PSGL - 1
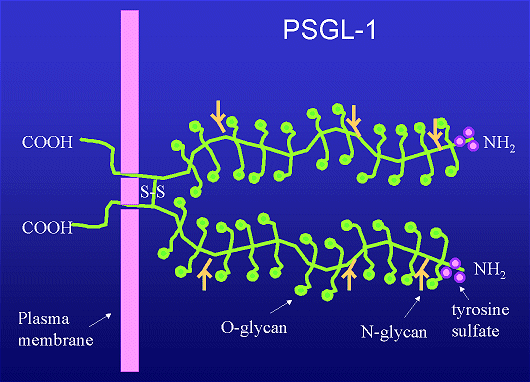
P-selectin glycoprotein ligand-1 is a 240 kDa homodimer consisting of two 120kDa polypeptide chains. PSGL-1 is constitutively expressed on all leukocytes. PSGL-1 is primarily found on the tips of the microvilli. PSGL-1 can bind to P-selectin on the endothelium when decorated with appropriate sugars. The structure of functional PSGL-1 includes the sialyl-Lewisx component. The PSGL-1 gene encodes a transmembrane polypeptide rich in proline, serine and threonine residues typical of mucin-type glycoproteins. The O-linked glycans displayed by PSGL-1 must undergo two specific post-translational modifications in order for it to function as a counter-receptor for P-selectin: a(1,3) fucosylation and a(2,3) sialylation. Bonds between P-selectin and PSGL-1 primarily mediate the rolling phase of the adhesion cascade.
View various information about this molecule at the National Center for Biotechnology Information. |
Search similar protein and nucleotide sequences between various
species using BLAST (Basic Local Alignment Search Tool)
|
| Read the following categorized abstracts or link to Medline to conduct your own query.
|
Comment on this page's content or view comments made by others!