Selectins
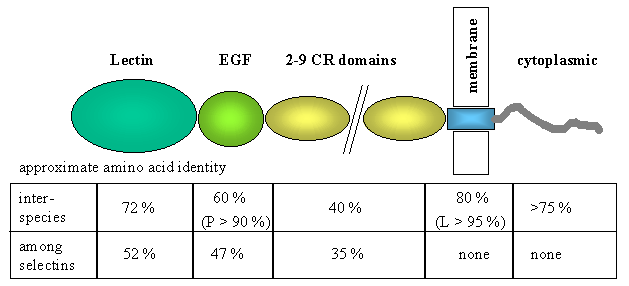
Selectins are a family of transmembrane molecules, expressed on the surface of leukocytes and activated endothelial cells. Selectins contain an N-terminal extracellular domain with structural homology to calcium-dependent lectins, followed by a domain homologous to epidermal growth factor, and two to nine consensus repeats (CR) similiar to sequences found in complement regulatory proteins. Each of these adhesion receptors is inserted via a hydrophobic transmembrane domain and possesses a short cytoplasmic tail. The initial attachment of leukocytes, during inflammation, from the blood stream is afforded by the selectin family, and causes a slow downstream movement of leukocytes along the endothelium via transient, reversible, adhesive interactions called leukocyte rolling. Each of the three selectins can mediate leukocyte rolling given the appropriate conditions.
L-selectin is the smallest of the vascular selectins, and can be found on most leukocytes. P-selectin, the largest selectin, is expressed on activated platelets and endothelial cells primarily. E-selectin is expressed on activated endothelium with chemically or cytokine-induced inflammation.
L-selectin | P-selectin | E-selectin | Selectin Ligands
3-D Structures of Selectins: Search results of the Protein Data Bank at Brookhaven National Laboratory using the pdblite search query to locate the structures of selectins. From the list of possible selectin molecules, choose the one of interst, and choose "View/Analyze/Save Macromolecule." Then, choose a method to view the molecule. RasMol is the suggested option for 3-D visualization.
Comment on this page's content or view comments made by others!