Selectin Ligands
During the inflammatory response, leukocyte adhesion to endothelial cells is controlled by the binding of vascular selectins to complementary carbohydrate ligands. All known selectin ligands are transmembrane glycoproteins which present oligosaccharide structures to the selectins. Transient bond formations between the selectins and their ligands mediate the early steps of the adhesion cascade. All three selectins can recognize glycoproteins and/or glycolipids containing the tetrasaccharide sialyl-Lewisx (sialyl-CD15). This tetrasaccharide is found on all circulating myeloid cells and is composed of sialic acid, galactose, fucose, and N-acetyl-galactosamine. It is unclear how selectins achieve specific interactions with ligands, given this common carbohydrate recognition.
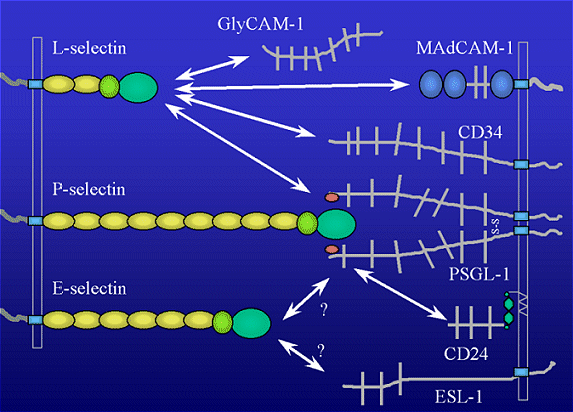
PSGL-1 (P-selectin Glycoprotien Ligand) has been characterized as a ligand for P-selectin. PSGL-1 is a glycoprotein expressed on blood cells and contains the sialyl-Lewisx tetrasaccharide. Another P-selectin ligand is CD24, which appears to be important for tumor cell binding to P-selectin. For L-selectin, four possible ligands have been identified: GlyCAM-1 (Glycosylation-Dependent Cell Adhesion Molecule), CD34, and MAdCAM-1 (Mucosal Addressin Cell Adhesion Molecule), and PSGL-1. Evidence suggests that both sulfate and sialic acid in an a(2,3) linkage are essential to L-selectin ligand activity. Although specific ligands for E-selectin are not yet known, candidate molecules include fucosylated, sialyated oligosaccharides found as components of glycoprotein and glycolipid molecules.
GlyCAM-1 | CD34 | MAdCAM-1 | PSGL-1
Comment on this page's content or view comments made by others!